Dermatophytes are a group of pathogenic fungi (predominantly from the Trichophyton and Microsporum genera) that infect the nails, skin, and hair. Dermatophyte infections are common in both humans and animals, and infection can spread from one species to the other.
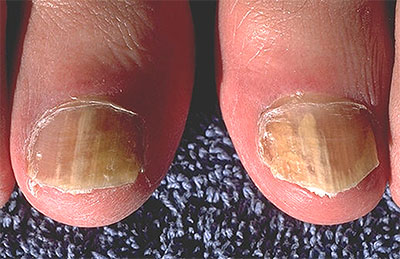
Dermatophyte infection of the nails results in onychomycosis (fungal nail infection), while dermatophyte infection of the hair and scalp results in tinea capitis (ringworm of the scalp). Onychomycosis is a widespread disease that is particularly common in the elderly and has the greatest potential for severe consequences in diabetic patients. Tinea capitis is a pediatric disease that disproportionately affects children from socioeconomically disadvantaged backgrounds and sub-Saharan African descent. For both diseases, rapid diagnosis is critical for promptly initiating the correct antifungal treatment.
In animals, dermatophyte infection of the fur and skin results in ringworm. The disease is especially prevalent in kittens and is a major challenge in animal shelters. Every year, approximately 3.2 million cats enter U.S. animal shelters, and every cat receives some form of health exam at intake, which includes an evaluation for ringworm infection.
Diagnostic challenges
The current gold standards for diagnosis of onychomycosis and tinea capitis are microscopy and fungal culture. Microscopy requires a high degree of skill, is expensive and is most often done on samples that have been shipped to reference laboratories, which means results take several days. Fungal culture also requires sending a sample to a reference lab, and results are generally not available for 2-4 weeks. Consequently, many doctors diagnose the infection based on the patient’s clinical presentation alone. But because the symptoms of onychomycosis and tinea capitis are difficult to distinguish from other disorders, many patients with dermatophyte infections fail to receive appropriate anti-fungal treatment, while other patients without dermatophyte infections unnecessarily receive anti-fungal drugs.
It is critical to get the diagnosis and treatment plan right. Failure to provide anti-fungal treatment to patients with dermatophyte infections can result in more severe disease for the patient and continued disease transmission in the community. Conversely, incorrectly prescribing anti-fungal drugs to patients without dermatophyte infections can result in unnecessary risk exposure, because the particular anti-fungals used to treat these diseases are oral drugs that must be taken for weeks and have potential for serious systemic side effects.
In veterinary practice, animals are commonly diagnosed via Wood’s lamp examination and/or fungal culture. Both approaches require specialized skill and training. Furthermore, the long wait for fungal culture results adds additional patient management, clinic workflow, and financial challenges.
The consequences of a misdiagnosis are serious: releasing an animal from treatment and quarantine early (or failing to accurately detect infection at intake) could result in shelter outbreaks and zoonotic transmission to pet owners. Access to rapid ringworm test results would be a gamechanger, leading to fewer uninfected animals unnecessarily quarantined (or euthanized) at intake and a more efficient exit from quarantine for treated animals. This would reduce shelter costs, free up staff time, increase bedspace turnover, and improve socialization and adoptability for affected cats and dogs.
DxDiscovery solution
Our solution is to produce a rapid, point-of-care diagnostic for dermatophyte infection of the hair, skin, and nails that is accurate, affordable, and suitable for use in resource-limited settings – including private veterinary practices and animal shelters. For human patients, the goal is to correctly diagnose onychomycosis and tinea capitis during the patient’s first clinic visit, so that patients can receive prompt, appropriate treatment and return to their daily lives. For veterinary patients, the goals are: i) to promptly diagnose ringworm (thereby protecting pet owners and other animals from infection), and ii) to efficiently detect when clearance of infection has occurred and quarantine can safely end (thereby accelerating shelter animals towards adoption and already-housed pets [and their owners] towards normalcy).
The target population will be all patients who present to the clinic with symptoms of potential onychomycosis, tinea capitis, or ringworm. The product will be a low-cost lateral flow immunoassay (similar to an at-home COVID-19 antigen test) that will detect material made by the pathogenic fungus in nail, hair, or skin swab specimens and will provide easy-to-read “yes/no” results in less than 20 minutes. Furthermore, the assay is designed to be run without any specialty equipment and the results can be read with the naked eye (no UV light source or camera needed).
Market opportunity
In the United States, approximately 11 million adults are affected by onychomycosis every year. An additional 3 million children in the U.S. are affected annually with tinea capitis.
In veterinary practice, over 3 million cats enter animal shelters each year – every one of which is examined for ringworm.
R&D funding
DxDiscovery has been awarded Small Business Innovation Research (SBIR) Phase I and Phase II grants from the National Institute of Biomedical Imaging and Bioengineering for development of the dermatophyte diagnostic.
For more information, please contact us: ContactUs@DxDiscovery.com